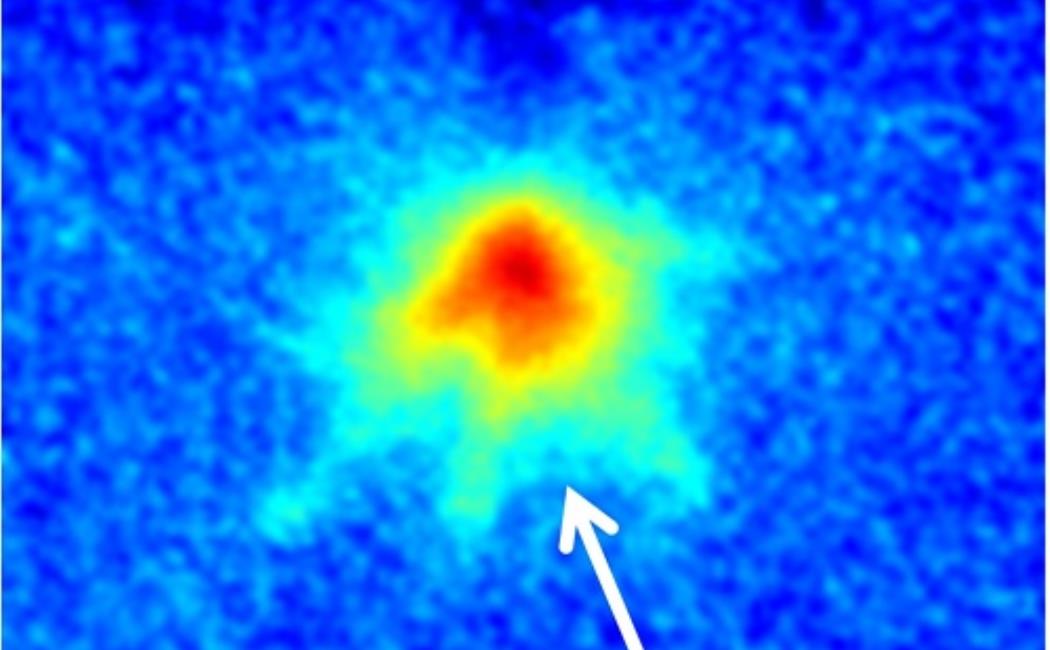
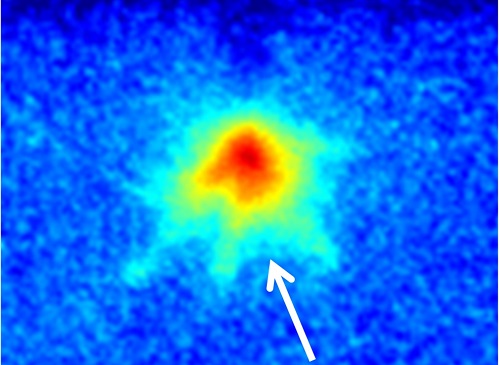
A thin, pancake-shaped plasma cloud formed at oil–water interfaces can be used to synthesize exotic nanomaterials.
Reproduced with permission from reference 1.© 2016 AIP
Oil and water do not mix, but a KAUST team has exploited the distinct interfaces between these substances to make plasma generation in liquids more efficient1,2. This approach holds promise for high-yield synthesis of nanomaterials from liquid reagents or for controlled elimination of water-borne parasites.
Familiar versions of plasmas in neon signs and TV displays use stable, charged particles in a gaseous state. But when produced in water from nanosecond bursts of electricity, positive ions in the plasma cool down significantly compared to energetic hot electrons. The resulting non-thermal discharges are capable of transferring energy to or from surrounding molecules, making them potential influencers of chemical reactions.
Ahmad Hamdan, a postdoctoral researcher with Min Suk Cha, is working to broaden the impact of in-liquid plasmas by lowering typical breakdown voltage requirements. Initially, he and his coworkers injected tiny gas bubbles into liquids to disrupt the usual electric field and create regions of amplified intensity to rip charges apart. Unfortunately, this strategy tended to trap the hot electrons within the bubbles, isolating them from potential chemical targets.
These investigations, however, revealed that changes in dielectric permittivity, a parameter that affects how electric fields propagate in materials, played a key role in bubble-based field enhancement. Hamdan realized that this phenomenon could be reproduced by dipping an electrode into two liquids with different responses to electric fields—a layer of low-dielectric heptane oil on top of water, for instance.
By optimizing the electrode’s position within the oil-water interface, Hamdan found he could generate plasmas in the liquids with 100% probability at lower than normal voltages. Moreover, the plasma propagates along the interface with a five-fold enhancement in discharge volume.
“This is interesting because the plasma is capable of decomposing both liquids without eroding the electrode,” notes Hamdan. “This gives us the flexibility to try and turn a wide range of substances into novel nanomaterials.”

An image of the oil–water interface.
© 2017 Min Suk Cha
As a demonstration, the team replaced the heptane layer with a hexamethyldisilazane oil containing silicon, nitrogen and hydrocarbon atoms. Applying the nanosecond electrical discharges to this oil-water interface produced organosilicon nanoparticles at quick rates—several milligrams per minute—safely confined inside the liquid medium. Further post-processing yielded species stable enough for high-temperature microelectronic devices.
Important to this work was the transmission electron microscopy provided by Dalaver Anjum at the Imaging and Characterization Core Lab of KAUST, which proved crucial for establishing relationships between silica nanoparticles of different sizes and their dielectric properties. Furthermore, a special electron prism on the microscope provided element-by-element breakdown of individual nanoparticle compositions.
The applications of this work are broad. “We have demonstrated only part of the potential feasible applications,” says Cha. “This novel idea can be extended to water purification, biomedical applications, and upgrade of low-quality liquid fuels; we will continue to work to make these applications tangible.
References
Hamdan, A. & Cha, M. S. Low-dielectric layer increases nanosecond electric discharges in distilled water. AIP Advances 6, 105112 (2016).| article
Hamdan, A., Halim, R. A., Anjum, D. & Cha, M. S. Synthesis of SiOC:H nanoparticles by electrical discharge in hexamethyldisilazane and water. Plasma Processes and Polymers,e1700089 (2017).| article
Read this article and more in KAUST Discovery where it was originally published.